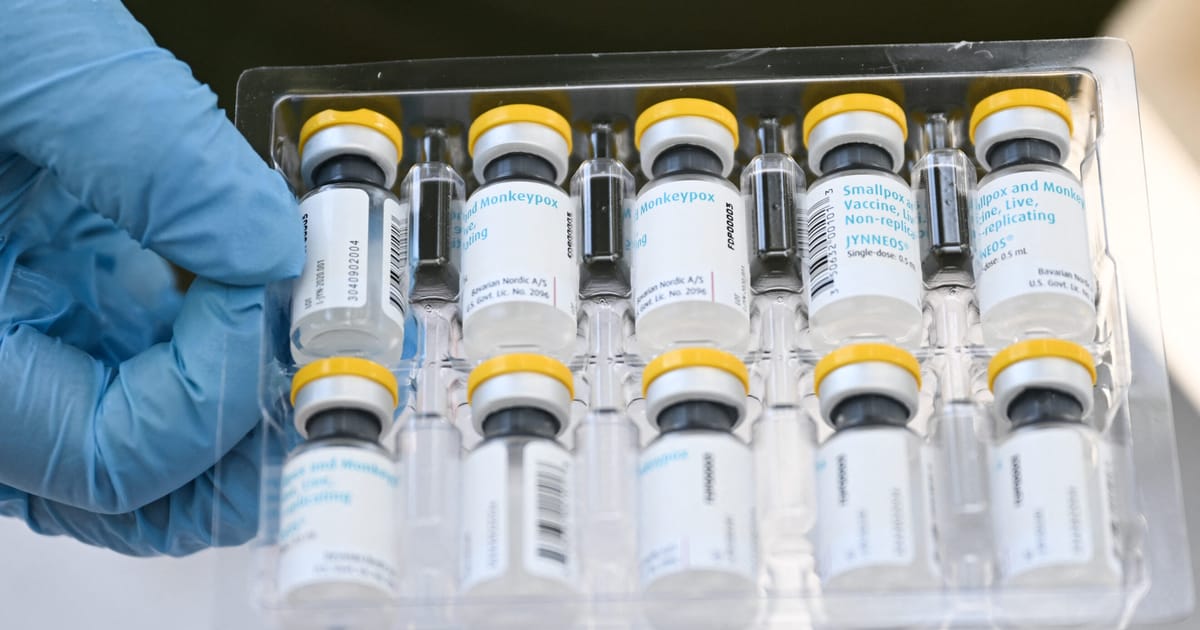
The World Health Organization (WHO) announced on Monday that it has approved Bavarian Nordic’s mpox vaccine for adolescents aged 12 to 17, recognizing this age group as particularly vulnerable to outbreaks of the disease that has raised global alarm.
In a statement, the WHO confirmed that it granted prequalification for the Jynneos vaccine for adolescents on October 8. The organization had previously declared mpox a global public health emergency for the second time in two years this past August, following the spread of a new variant from the Democratic Republic of Congo to neighboring countries.
In September, the WHO approved the use of the Jynneos vaccine for adults, facilitating access for severely affected African nations. Mpox, a viral infection characterized by flu-like symptoms and pus-filled skin lesions, poses significant risks to children, adolescents, and individuals with weakened immune systems.
The WHO’s recent decision aligns with the European Union’s approval of the vaccine for adolescents in September. Meanwhile, the Danish biotech firm is set to conduct a clinical trial to evaluate the vaccine’s safety in children aged two to 12, potentially expanding its application. This trial, partially funded by the Coalition for Epidemic Preparedness Innovations, is anticipated to begin in October.
In the United States, the Food and Drug Administration (FDA) has approved Bavarian Nordic’s vaccine for adults aged 18 and older but issued Emergency Use Authorization for adolescents during the 2022 mpox outbreak.
Additionally, Japan’s KM Biologics produces another mpox vaccine, LC16, which is approved for use in children, though it requires a specialized needle for administration. Bavarian Nordic has not yet commented on the recent prequalification.