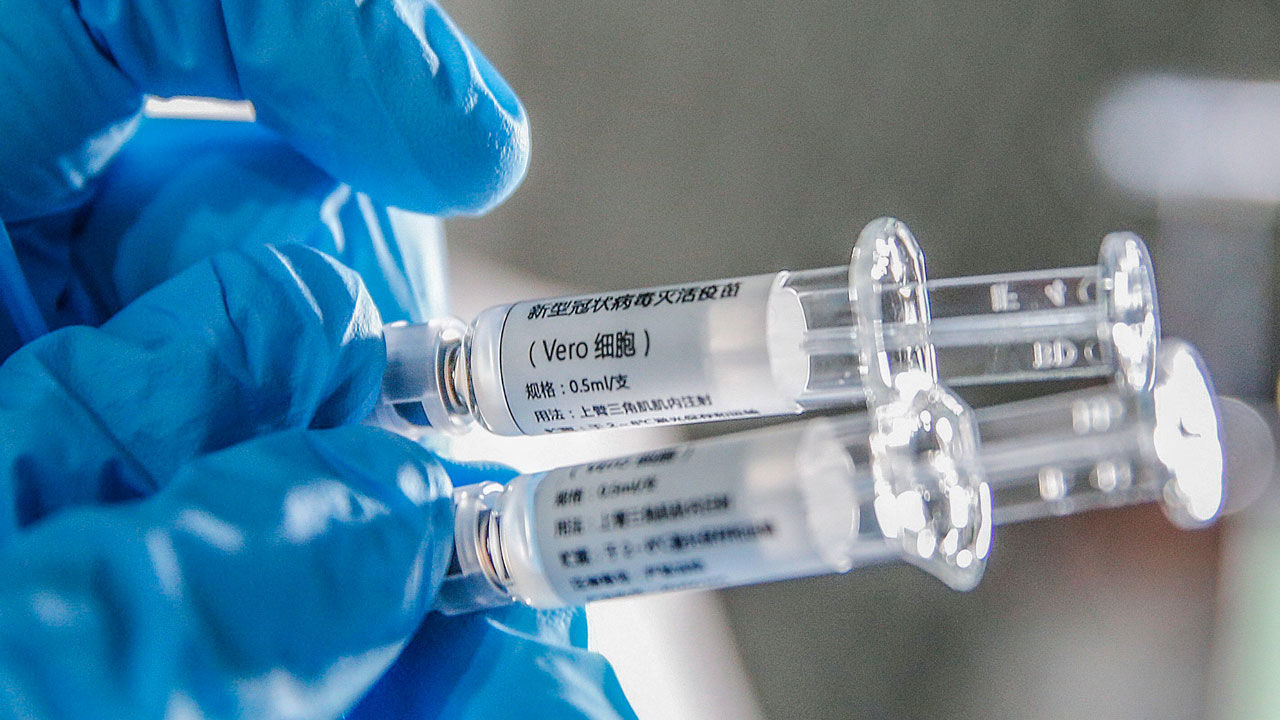
China approves its first state-owned coronavirus vaccine. On Thursday, health regulators stated that conditional approval is given to the company named Sinopharm. It is a two-dose vaccine that is the first approved for general use in China.
Pakistan pre-booked 1.1 million doses of the vaccine.
Asad Umar– Planning and Development Minister
The deputy commissioner of the National Medical Products Administration, Chen Shifei, expressed in a news conference,
“Conditional approval means that research is still ongoing, the company will be required to submit follow-up data as well as reports of any adverse effects after the vaccine is sold on the market.”
“The company must continuously update the vaccine’s instructions, labels and report to the agency,” Shifei said.
After the cabinet’s approval for the procurement, Pakistan pre-booked 1.1 million doses of the vaccine, said the Planning and Development Minister Asad Umar.
The aforementioned vaccine was developed by the Beijing Institute of Biological Products, a subsidiary of the state-owned corporation Sinopharm. The conglomerate announced that preliminary data from last-stage trials had shown 79.3% efficacy, on Wednesday.
The virus was grown in a lab and then killed, the germ is then injected into the body to generate an immune response, which makes it an inactive vaccine.
The concluding evidence of its effectiveness will depend on publication of more data.
Our Latest Updates
- PM Shehbaz Orders Urgent Probe into Private Hajj Quota Reduction
- Trump Slaps Tariffs on Penguin-Populated Islands
- Baba Vanga’s Chilling 2025 Predictions: Is This the Beginning of Humanity’s Downfall?
- Illegal Arms Trade Surges Along Afghanistan-Pakistan Border Despite Taliban Crackdown
- WhatsApp Rolls Out Advanced Chat Privacy Controls in Latest Update