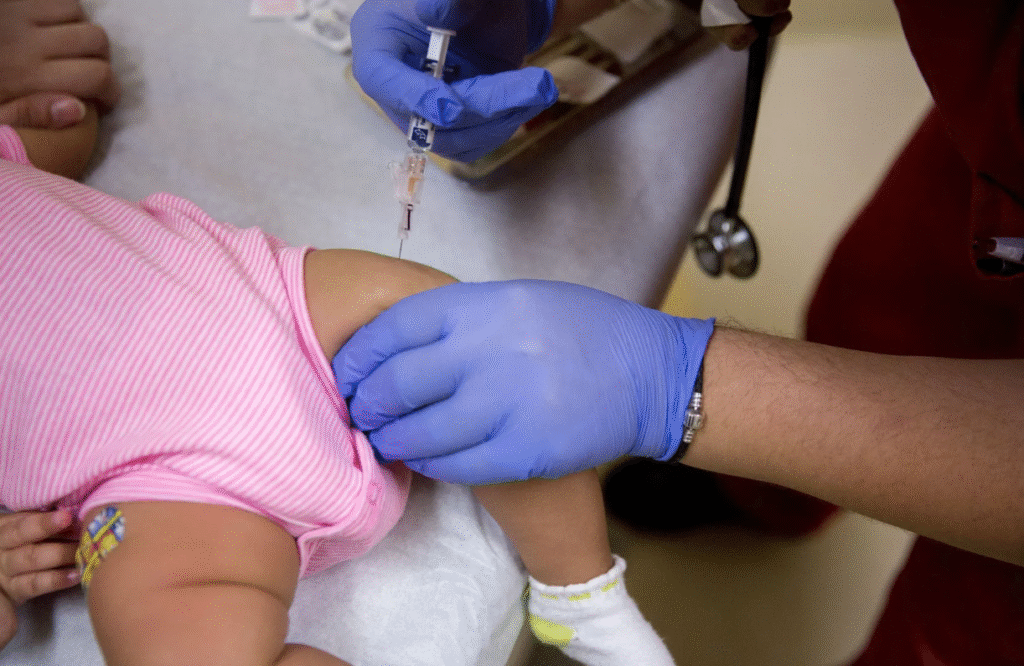
The US Centers for Disease Control and Prevention (CDC) has ended the longstanding policy of vaccinating all newborns against hepatitis B. Moving forward, the decision will be left to parents in consultation with healthcare providers. Infants born to mothers who test negative for hepatitis B are no longer automatically scheduled to receive the birth dose.
This policy shift follows recommendations from the Health Secretary’s vaccine advisory panel. The panel suggested that only infants born to mothers who test positive for hepatitis B, or whose status is unknown, should receive the vaccine at birth. The CDC approved this approach, marking a major change in national healthcare policy.
Parents Now Play a Larger Role in Vaccination Decisions
Parents who choose to delay vaccination are advised to wait at least two months before giving their child the first dose of hepatitis B vaccine. This change allows for individualized decision-making but also raises concerns among experts about potentially increasing infants’ exposure to the virus.
Since 1991, US health officials have recommended universal hepatitis B vaccination for newborns. The program previously involved three doses, with the first administered shortly after birth. Universal vaccination contributed to a nearly 90% drop in hepatitis B infections, from 9.6 cases per 100,000 individuals before the vaccine to about one per 100,000 by 2018.
Expert Concerns Over Increased Risk
Medical professionals warn that removing a firm federal mandate could lead more families to skip vaccination. Hepatitis B is a serious virus that can cause chronic liver disease and is spread through blood, semen, certain body fluids, and close contact with infected individuals.
Dr. Emily Landon, an infectious diseases expert, criticized the decision, stating it ignores scientific evidence and may undermine health improvements achieved through decades of vaccination. She emphasized the importance of science-based policies to protect infants from preventable diseases.
Policy Implications for Healthcare Providers
The new recommendation affects both health insurance coverage and clinical guidance. Physicians must now interpret the risk individually for each newborn, often relying on antibody testing to determine if a subsequent vaccine dose is necessary.
The CDC continues to evaluate guidance on follow-up antibody testing to ensure infants remain protected. Meanwhile, public health officials stress the importance of parental consultation with qualified healthcare providers before making vaccination decisions.